Blinatumomab (BLINCYTO) Prescribing Information | Hong Kong Dengyue Medicine
BLINCYTO® is the world’s first approved BiTE® immuno-oncology therapy, targeting the CD19 surface antigen on B cells. BiTE® molecules help the immune system detect and target malignant cells by bringing T cells—a type of white blood cell capable of killing cells recognized as threats—into contact with cancer cells. By positioning T cells near cancer cells, they can inject toxins and trigger cancer cell death (apoptosis). The potential of BiTE® immuno-oncology therapy for treating various cancers is currently under investigation.
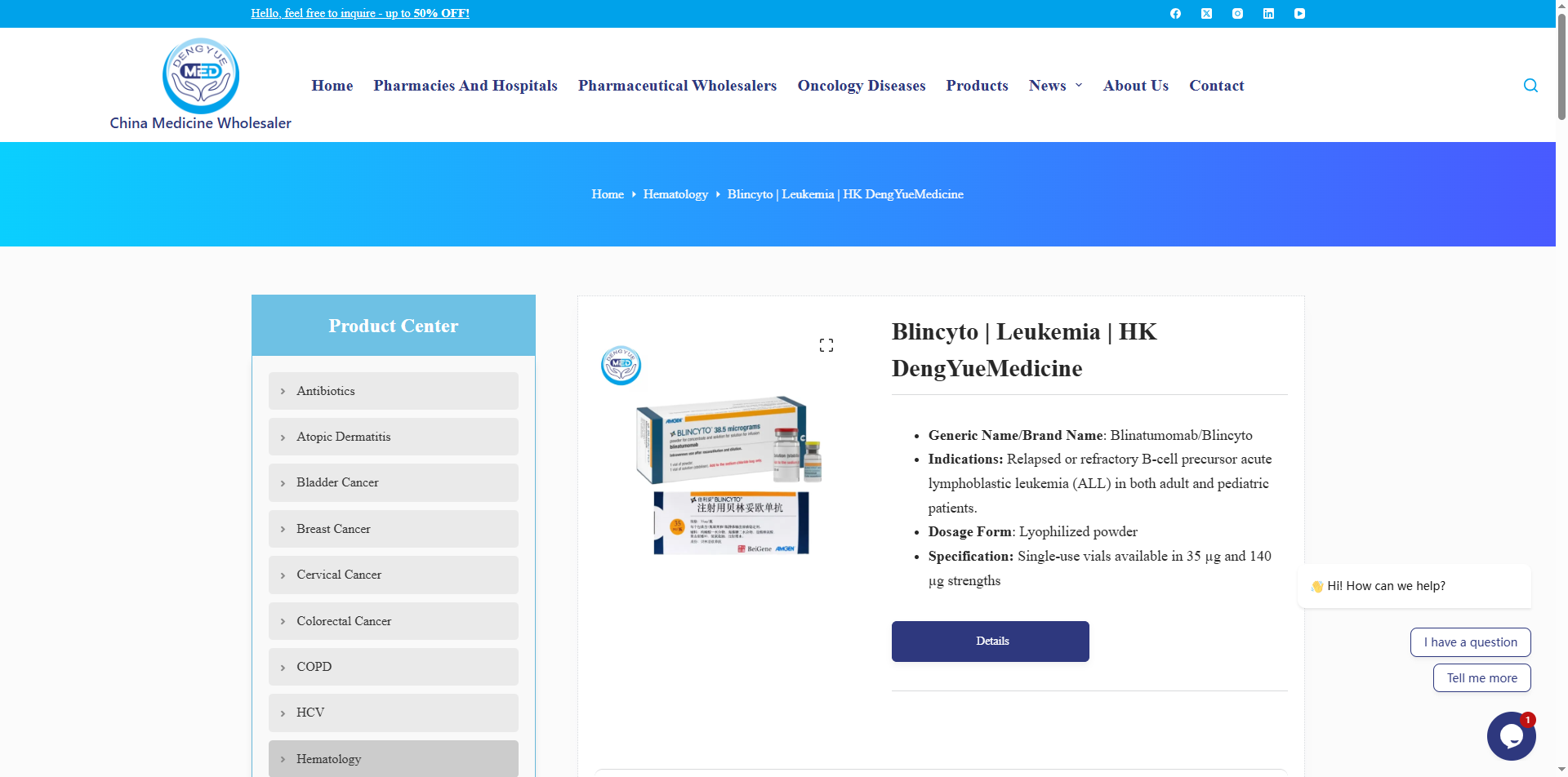
Drug Name / Brand Name: Blinatumomab (BLINCYTO® / Bei Li Tuo)
Dosage Form / Route of Administration: Intravenous infusion
Drug Type: Antibody
Formulation: Lyophilized powder for injection
Indications
BLINCYTO® (blinatumomab) is indicated for the treatment of adults with relapsed or refractory precursor B-cell acute lymphoblastic leukemia (ALL). It is suitable for the treatment of CD19-positive B-cell precursor ALL in adults and children aged one month and older, including:
-
Philadelphia chromosome-negative disease in multiple cycles of consolidation therapy
-
Minimal residual disease (MRD) ≥ 0.1% during first or second complete remission
-
Relapsed or refractory disease
Efficacy
Blinatumomab was approved by the FDA on December 3, 2014, for the treatment of relapsed Philadelphia chromosome-negative B-cell ALL.
Blinatumomab is a bispecific antibody targeting CD19 on B cells and CD3 on T cells, bringing T cells into close proximity to cancer cells to exert cytotoxic effects. After binding to T cells, blinatumomab further activates T cell signaling pathways, inducing expression of CD69 and CD25, upregulating adhesion molecules (CD2), transiently releasing inflammatory cytokines, activating T cells, and promoting T cell proliferation.
In December 2020, blinatumomab was first approved in China for the treatment of adult patients with relapsed or refractory precursor B-cell ALL.
On August 19, 2021, BeiGene announced the commercial launch of BLINCYTO® (blinatumomab injection), making it available in multiple hospitals nationwide for patient prescriptions.
On April 29, 2022, the National Medical Products Administration (NMPA) of China approved a new indication for blinatumomab injection, expanding its use to treat both adult and pediatric patients with relapsed or refractory precursor B-cell ALL.
Adverse Reactions
Common adverse reactions include:
-
Cytokine release syndrome (CRS)
-
Neurological toxicities, such as seizures or altered consciousness
-
Fever, headache, hypotension, nausea, vomiting
During treatment, healthcare providers closely monitor patient responses and adjust the treatment regimen as necessary.
Precautions
-
Initial treatment should be administered in a medical facility equipped with emergency care to manage potential severe adverse reactions.
-
Avoid concomitant use of other immunosuppressants unless deemed necessary by a physician.
-
Live vaccines should be avoided during treatment.
Note: This information is intended for medical research and educational purposes and does not constitute medical advice. For specific guidance on medication use, please consult your treating physician. For professional import and export services, you may contact Hong Kong Dengyue Pharmaceutical.
- Art
- Causes
- Crafts
- Dance
- Drinks
- Film
- Fitness
- Food
- Jeux
- Gardening
- Health
- Domicile
- Literature
- Music
- Networking
- Autre
- Party
- Religion
- Shopping
- Sports
- Theater
- Wellness


