The Role of CRISPR and AAV in Duchenne Gene Repair
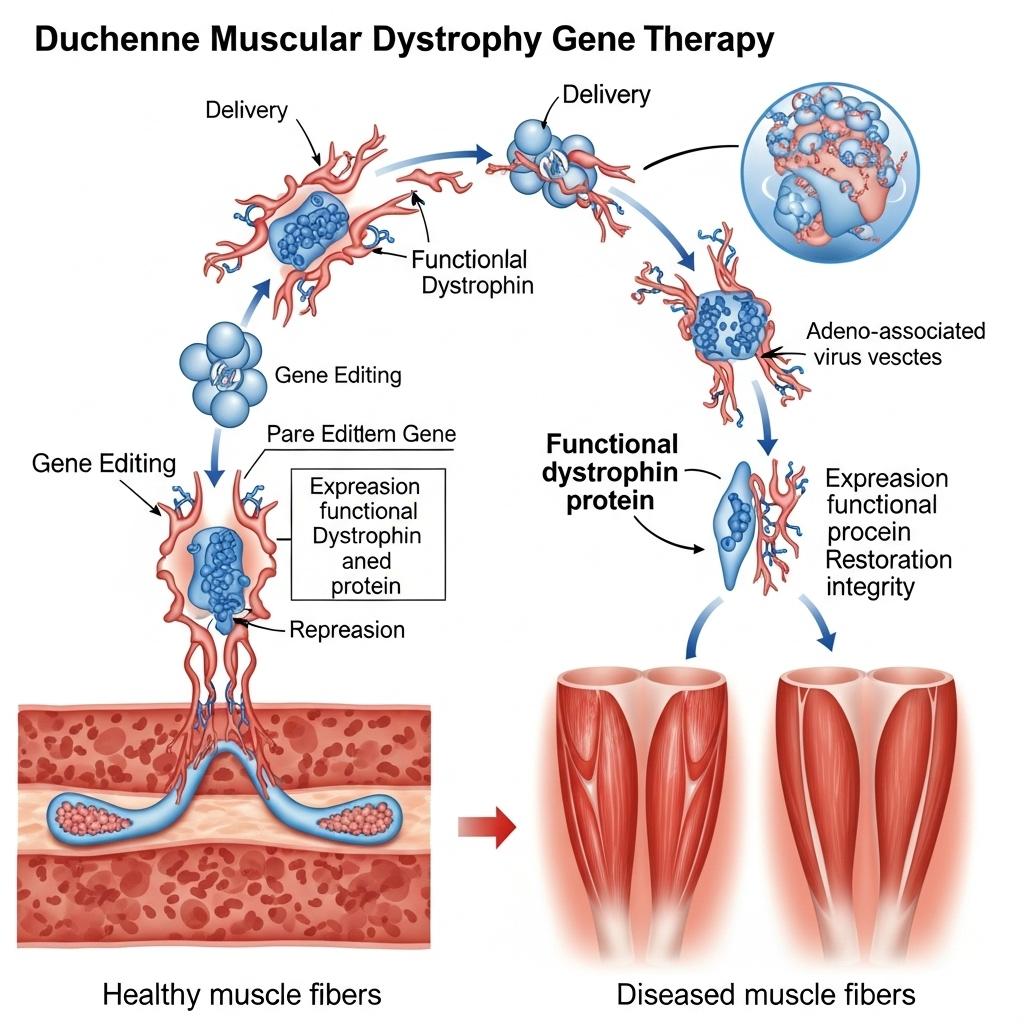
Duchenne Muscular Dystrophy (DMD) is a rare, progressive neuromuscular disorder that leads to severe muscle degeneration and early mortality. Affecting roughly 1 in 3,500 to 5,000 male births globally, DMD stems from mutations in the DMD gene, which encodes dystrophin, a protein critical for muscle fiber integrity. Without dystrophin, muscle cells become fragile and eventually die, leading to progressive weakness and loss of motor function.
Until recently, treatment strategies were limited to corticosteroids, supportive care, and symptom management. Today, gene therapy is emerging as a transformative option offering the potential to alter the natural course of DMD.
Request a sample copy of the CI report at: https://www.datamintelligence.com/download-sample/duchenne-muscular-dystrophy-treatment-market
The Genetic Basis of DMD
The DMD gene is the largest known human gene, making it difficult to replace or repair. Most DMD cases involve deletions, duplications, or nonsense mutations that disrupt dystrophin production. This protein acts as a structural stabilizer between the cytoskeleton and extracellular matrix in muscle tissue.
Loss of function leads to:
1. Muscle cell fragility
2. Chronic inflammation
3. Fibrosis and fat replacement of muscle
4. Respiratory and cardiac decline over time
Boys typically show symptoms by age 2–5, lose ambulation in their early ***s, and may develop life-threatening cardiac or pulmonary complications in their twenties.
Gene Therapy: A New Era in DMD Treatment
The core goal of gene therapy in DMD is to restore dystrophin expression or modify the underlying mutation to reduce disease severity. Several gene-based platforms are currently under investigation or have entered the market, including:
1. Micro-dystrophin Gene Replacement Therapy
Due to the large size of the dystrophin gene (~2.4 Mb), full-length gene delivery is not feasible with common vectors. Instead, researchers have developed micro-dystrophin constructs, smaller versions that retain key functional domains.
Delivery platform: Adeno-associated *** (AAV) vectors
Target: Skeletal and cardiac muscle
Example: Delandistrogene moxeparvovec (Elevidys), conditionally approved for eligible DMD patients
While early data are promising, challenges remain regarding immune responses, durability, and long-term safety.
2. Exon Skipping Therapies
This approach uses antisense oligonucleotides (AONs) to "skip" faulty exons during mRNA splicing, enabling production of a partially functional dystrophin protein.
Approved agents: Eteplirsen (exon 51), Golodirsen (exon 53), Viltolarsen. Not curative, but shown to slow progression in select mutation subtypes
3. CRISPR/Cas9 Gene Editing
Still in early stages, CRISPR-based approaches aim to correct the mutation at the DNA level. Preclinical models show potential, but delivery, off-target effects, and scalability are key hurdles.
Clinical Trials and Regulatory Milestones
Ongoing clinical trials are evaluating safety, durability, and functional outcomes associated with gene therapy in DMD.
Key focus areas include:
1. Functional motor improvements: North Star Ambulatory Assessment (NSAA), 6-minute walk test
2. Biomarker ***ysis: Dystrophin protein expression via Western blot or immunohistochemistry
3. Safety signals: Liver enzyme monitoring, immune reactions, vector-related inflammation
4. While gene therapies like Elevidys have received accelerated approvals, confirmatory trials are essential to secure full approval and broader access.
Read the full CI Insights report: https://www.datamintelligence.com/strategic-insights/duchenne-muscular-dystrophy-gene-therapy-competitive-edge-insights
Challenges and Future Perspectives
Despite unprecedented promise, several challenges must be addressed:
1. Immune response: Pre-existing antibodies against AAV vectors can reduce therapy effectiveness
2. Age and weight limitations: Dosing is limited by vector capacity and safety considerations
3. One-time dosing limitation: Repeat administration may be restricted due to immune sensitization
4. Long-term durability: Need for ongoing studies to track efficacy beyond 3–5 years. Researchers are also exploring next-gen vectors, non-*** delivery systems, and re-dosing strategies to overcome current barriers.
5. Equity and Access: High costs, infrastructure requirements, and eligibility restrictions pose access challenges in many regions. Collaboration between regulators, patient advocacy groups, and industry is essential to ensure:
* Expanded clinical trial access
* Equitable post-approval rollout
* Global manufacturing and delivery capacity
About DataM Intelligence
DataM Intelligence 4Market Research LLP delivers real-time competitive intelligence across autoimmune, immunologic, and rare disease landscapes. We provide actionable insights across clinical pipelines, regulatory milestones, and commercial strategy for life sciences stakeholders.
🔗 Visit: www.datamintelligence.com
- Art
- Causes
- Crafts
- Dance
- Drinks
- Film
- Fitness
- Food
- Jogos
- Gardening
- Health
- Início
- Literature
- Music
- Networking
- Outro
- Party
- Religion
- Shopping
- Sports
- Theater
- Wellness


