Спонсоры
GO Treatment Market Growth Catalyzed by Novel IGF-1R Inhibitors and Targeted Therapies
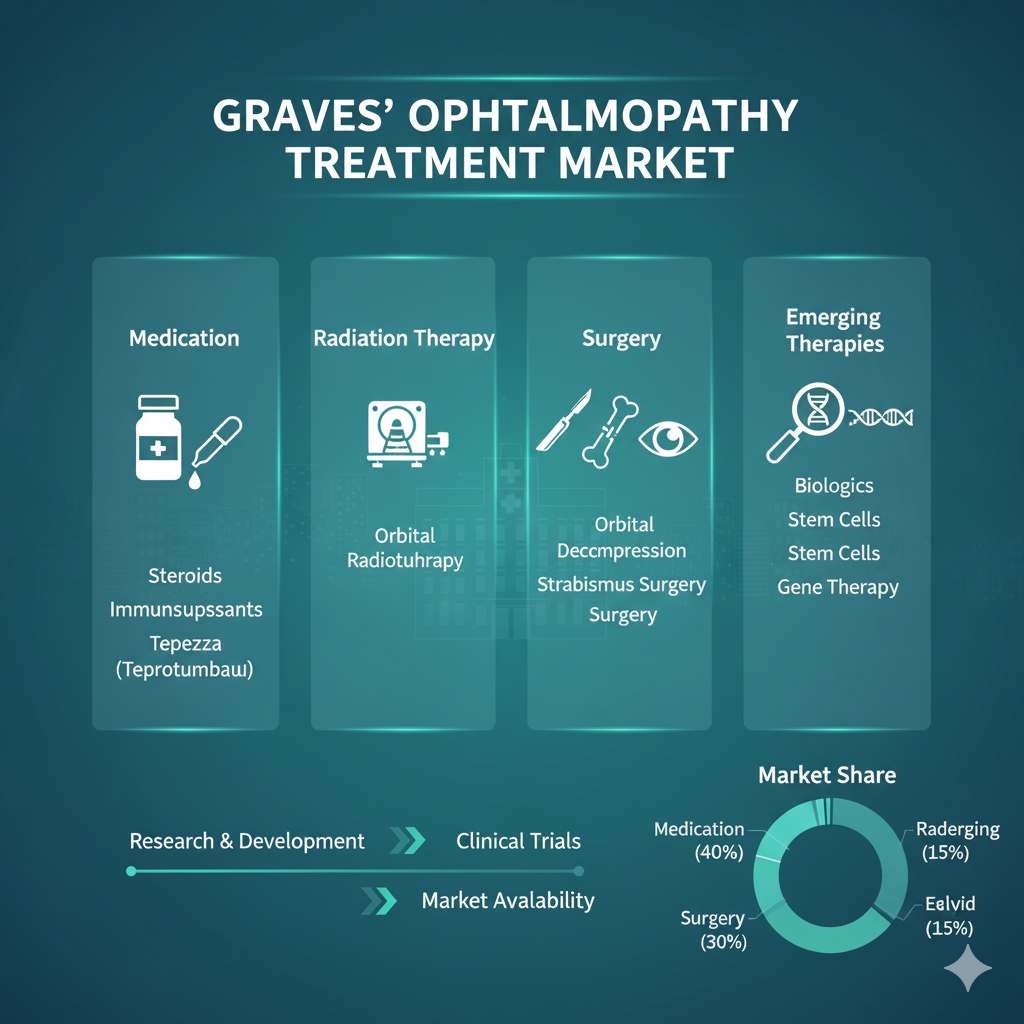
The global Graves ophthalmopathy (GO) treatment market growth was valued at US$ 2.09 billion in 2024 and is projected to reach US$ 3.01 billion by 2033, growing at a CAGR of 4.2% during the forecast period.
Download Sample PDF:
Market Overview
Graves ophthalmopathy, also known as thyroid eye disease (TED), is a serious autoimmune condition affecting the orbit around the eyes. It is clinically relevant in 25–50% of patients with Graves' disease and 2% of patients with chronic thyroiditis. The age-adjusted annual incidence of clinically relevant GO is 16 per 100,000 population in women and 2.9 in men.
Advancements in treatment options, a strong pipeline of products, increasing disease awareness, and growing global incidence are driving the market's transformation. Companies like Viridian Therapeutics, Immunovant, and Argenx are developing next-generation biologics aimed at reducing costs, increasing convenience, and improving safety profiles. These formats are expected to penetrate underserved and outpatient markets.
Market Segmentation
-
By Medication: Monoclonal Antibodies, Corticosteroids, Antithyroid Medications, and Others
-
By Disease Severity: Mild, Moderate, and Severe
-
By End-User: Hospitals, Specialty Clinics, Academic and Research Institutes, and Others
-
By Region: North America, Latin America, Europe, Asia Pacific, Middle East, and Africa
Regional Insights
-
North America: Expected to dominate the global Graves ophthalmopathy treatment market with a 42.73% share in 2024. The region's dominance is attributed to early adoption of advanced therapies, strong healthcare infrastructure, and significant R&D investment. The U.S. also leads in clinical trials and regulatory approvals.
Key Market Drivers
-
Pipeline Innovation: The rising number of pipeline products is significantly driving market growth. The approval of Tepezza (teprotumumab) in 2020 marked the first targeted biologic therapy and opened the floodgates for R&D investment in TED.
-
Tepezza’s limitations, such as IV-only administration, high cost, and side effects, have created an opportunity for innovation, accelerating the development of next-generation therapies that are subcutaneous, oral, or have novel mechanisms of action.
Leading Market Players
-
Amgen Inc.
-
Immunovant, Inc.
-
Viridian Therapeutics, Inc.
-
Argenx
-
Tourmaline Bio, Inc.
-
F. Hoffmann-La Roche Ltd
-
Sling Therapeutics
These companies focus on product development, acquisitions, and collaborations to advance therapies and expand market presence.
Recent Industry Developments
-
January 2025: Sling Therapeutics announced topline efficacy and safety data from the Phase 2b/3 LIDS trial of linsitinib in patients with active, moderate to severe TED.
-
May 2025: Viridian Therapeutics announced that the U.S. FDA granted Breakthrough Therapy Designation to veligrotug, the company’s lead anti-insulin-like growth factor-1 receptor (IGF-1R) drug candidate for the treatment of TED.
Conclusion
The Graves ophthalmopathy treatment market is poised for significant growth through 2033, driven by advancements in treatment options, a robust pipeline of next-generation biologics, and increasing disease awareness. With North America's dominance and rapid developments in pipeline therapies, the market is expected to witness continued innovation and expansion.





