North America Duchenne Muscular Dystrophy (DMD) Treatment Market to Surpass USD 8.84 Billion by 2033, Fueled by 20.2% CAGR
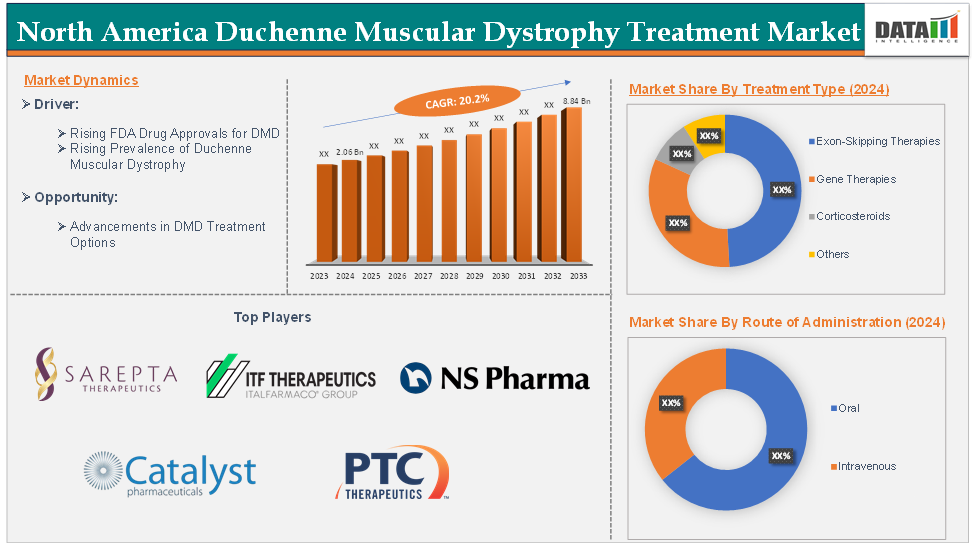
The North America Duchenne Muscular Dystrophy (DMD) Treatment Market was valued at US$ 2.06 billion in 2024 and is projected to reach US$ 8.84 billion by 2033, growing at a remarkable CAGR of 20.2% from 2025 to 2033.
DMD is a severe, progressive genetic disorder characterized by muscle weakness and degeneration, predominantly affecting young boys. Despite being rare, the high disease burden and lack of curative therapies have made this area a focus of intensive research, innovation, and policy support. North America leads the global landscape due to advanced healthcare infrastructure, strong clinical research, high diagnosis rates, and the presence of leading pharmaceutical companies driving innovation in gene therapies, exon-skipping drugs, and novel treatment modalities.
Get a Sample PDF Brochure of the Report (Use Corporate Email ID for a Quick Response):
Market Drivers
-
Regulatory Support & Orphan Drug Incentives – Accelerated approval pathways, priority reviews, and orphan drug designations encourage rapid development of new DMD treatments.
-
Emergence of Novel Therapies – Gene therapies, exon-skipping approaches, and HDAC inhibitors are expanding treatment options, shifting the paradigm from symptomatic management to disease-modifying strategies.
-
High Diagnosis Rates – Newborn screening initiatives and rising awareness improve early detection and timely intervention.
-
Growing R&D Investments – Pharmaceutical and biotech firms are investing heavily in clinical trials and advanced therapies, supported by strong venture funding.
-
Patient Advocacy & Support Networks – Foundations and advocacy groups drive awareness, research collaboration, and policy lobbying to expand access to therapies.
Market Restraints
-
High Treatment Costs – Gene therapies and exon-skipping drugs carry extremely high price tags, posing challenges for payers and patients.
-
Safety Concerns – Advanced therapies like gene treatments face scrutiny around long-term safety and potential adverse effects.
-
Access Inequality – While the U.S. leads in adoption, disparities in healthcare access and insurance coverage can delay or limit treatment availability.
-
Complex Regulatory Pathways – Despite accelerated approvals, stringent post-marketing surveillance and evolving safety requirements may slow widespread uptake.
Market Segmentation
By Treatment Type
-
Exon-Skipping Therapies
-
Gene Therapies
-
Corticosteroids
-
HDAC Inhibitors
-
Others
By Route of Administration
-
Oral
-
Intravenous
By End User
-
Hospitals & Clinics
-
Specialty Centers
-
Homecare Settings
Geographical Share
-
United States dominates the market due to advanced healthcare infrastructure, high diagnosis and screening rates, strong regulatory support, and reimbursement mechanisms. The U.S. also has the highest concentration of clinical trials and therapy launches, making it the center of innovation.
-
Canada is steadily increasing adoption, supported by a strong public healthcare system and growing alignment with U.S. regulatory approvals.
-
Mexico shows slower uptake but is gradually improving through collaborations, awareness campaigns, and evolving healthcare policies.
Overall, North America accounts for the largest share of the global DMD treatment market, reflecting both strong demand and a robust ecosystem for innovation.
Market Key Players
Major keyplayers in the Duchenne muscular dystrophy treatment market include Sarepta Therapeutics, Inc., ITF Therapeutics LLC, NS Pharma, Inc., Catalyst Pharmaceuticals, Inc., and PTC Therapeutics. Emerging players in the market include F. Hoffmann-La Roche Ltd, Capricor Therapeutics, Inc., REGENXBIO Inc., Solid Biosciences Inc., Wave Life Sciences, Genethon, and among others.
These players are leading advancements in gene therapy, exon-skipping, and novel oral treatments while engaging in mergers, acquisitions, and partnerships to expand their footprints.
Latest Developments
-
New Approvals – Recent FDA approvals of non-steroidal oral drugs and expanded indications for gene therapies have broadened treatment options across age groups.
-
Pipeline Expansion – Mid- and late-stage clinical trials are advancing, with promising exon-skipping and next-generation gene therapy candidates showing strong efficacy data.
-
Safety & Monitoring Updates – Post-approval safety reviews have led to more stringent monitoring of patients receiving gene therapies, reflecting the balance between innovation and patient safety.
-
Consolidation & Partnerships – Collaborations between biotech innovators and large pharmaceutical firms are accelerating commercialization and improving access strategies across the region.
-
Technological Innovations – Use of biomarkers, digital health tools, and real-world evidence is shaping clinical development and patient monitoring.
Market Outlook
The future of the North American DMD treatment market is defined by rapid innovation and growing patient access. Gene therapies are expected to become the cornerstone of treatment over the next decade, potentially transforming the disease outlook for patients. Exon-skipping therapies will continue to play an important role, particularly for mutation-specific cases.
Despite challenges such as high treatment costs and ongoing safety evaluations, strong regulatory support, R&D momentum, and patient advocacy are expected to sustain growth. By 2033, the treatment landscape will likely be more diversified, with improved affordability and broader availability across North America.
- Art
- Causes
- Crafts
- Dance
- Drinks
- Film
- Fitness
- Food
- משחקים
- Gardening
- Health
- בית
- Literature
- Music
- Networking
- לא משנה
- Party
- Religion
- Shopping
- Sports
- Theater
- Wellness


