Bioresorbable Implants Market Size, Share & Future Outlook
Market Size & Forecast
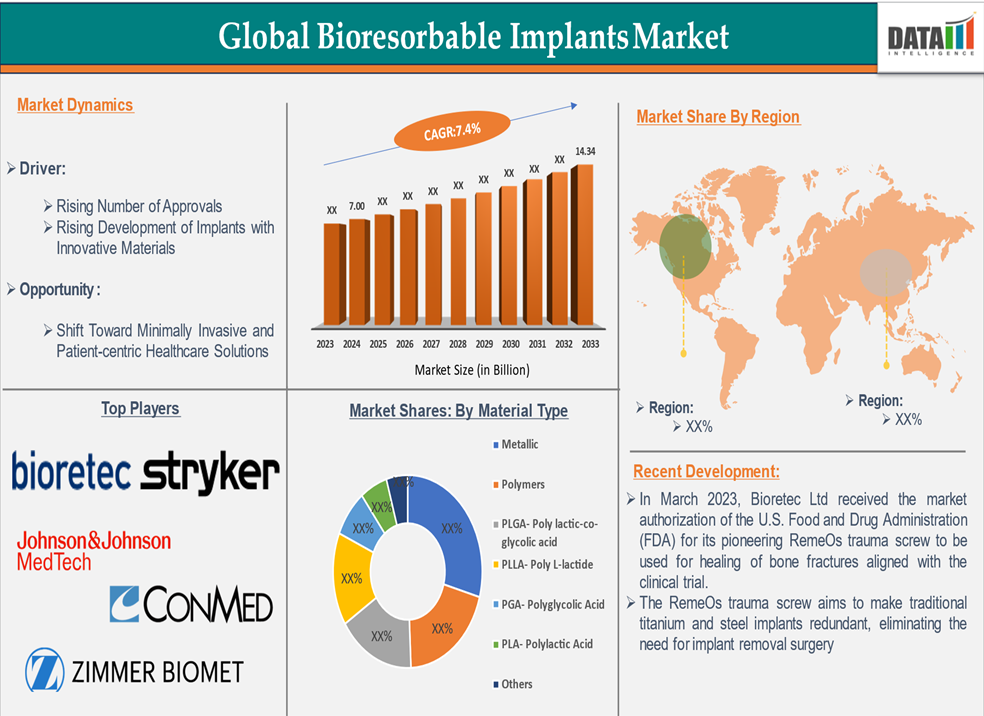
The bioresorbable implants market reached approximately US$7.00 billion in 2024. ***ysts forecast a rise to US$14.34 billion by 2033, representing a compound annual growth rate (CAGR) of 7.4% between 2025 and 2033.
Introduction & Definition
Bioresorbable implants are medical devices engineered from materials—such as hydrophilic or hydrophobic polymers and biodegradable metals—that safely dissolve in the body after fulfilling their function. ***gned for temporary support in orthopedic, tissue repair, cardiovascular, or ***-delivery applications, these implants reduce the need for removal procedures and the risk of chronic inflammatory responses.
Segmentation ***ysis
-
By Material Type:
PLGA (poly lactic-co-glycolic acid) dominates, thanks to its tunable degradation rates and regulatory approvals for use in devices such as screws and pins. Other polymers (PLLA, PGA, PLA) hold a significant share, while metallic alloys (notably magnesium-based) are gaining traction for their superior mechanical strength and controlled resorption. -
By Application:
Orthopedic procedures—fracture fixation, sports injuries—are the leading application area due to high incidence and growing demand for biodegradable devices. Cardiovascular uses are emerging, particularly in resorbable stents and scaffolding. -
By End User:
Hospitals capture the majority of usage, while ambulatory surgical centers are expanding their adoption rapidly.
Geographical Insights
-
North America commands a ~40% share of global demand in 2024. The region is supported by advanced healthcare infrastructure, high surgical volumes (e.g., 790k knee, 544k hip replacements annually in the U.S.), and fast regulatory pathways.
-
Europe remains strong with CE-marked product approvals and robust medical R&D.
-
Asia-Pacific, including Japan and India, is the fastest-growing region due to medical tourism, expanding healthcare access, and aging demographics.
-
Japan plays a significant role in biomaterials innovation; the broader Japanese biomaterials market was valued at US$12.1 billion in 2024 and is projected to reach nearly US$56.0 billion by 2035, though bioresorbables are a subset of that market.
To get a free sample report, click on https://www.datamintelligence.com/download-sample/bioresorbable-implants-market
Latest News & Industry Trends in the US & Japan
-
U.S.:
-
In March 2023, Bioretec received FDA approval for its RemeOs magnesium-based trauma screws, enabling U.S. commercialization.
-
Technological innovations in additive manufacturing and precision engineering are accelerating the development of patient-specific implants and integrating smart diagnostics (e.g., embedded sensors).
-
-
Japan:
-
Japan approved Abbott’s Absorb™ fully dissolving coronary stent, making it the only fully resorbable heart stent on the Japanese market.
-
Japanese R&D is driving high-performance metallic bioresorbables, particularly in magnesium alloys, in line with the country's booming biomaterials market.
-
Competitive Landscape
Key global players include
-
Bioretec Ltd.—pioneer in magnesium-based resorbable screws
-
Stryker Corp., Johnson & Johnson/DePuy Synthes, Zimmer Biomet—giants in orthopedic device innovation
-
CONMED, Syntellix AG, Smith+Nephew, and Evonik Industries are active in advanced polymers and bioceramics.
These companies are investing in R&D, securing regulatory clearances, forming strategic alliances, and optimizing distribution in key markets such as the U.S. and Japan.
Impact ***ysis
-
Clinical & Economic Benefits: Reduced need for removal surgeries enhances patient recovery and lowers overall treatment costs.
-
Patient Satisfaction: The Temporary nature of resorbable implants improves treatment outcomes and reduces long-term complications.
-
Regulatory Evolution: Approvals like the FDA’s for magnesium-based implants set a precedent for faster adoption.
-
Demographic Drivers: Aging populations (e.g., 56 million Americans aged 65+ in 2022) mean rising demand for bioresorbable solutions.
Key Developments
-
Jan 2025: CE mark approval granted in Europe for Bioretec’s RemeOs trauma screw, paving the way for global rollout.
-
Mar 2023: FDA grants market authorization for the U.S. launch of RemeOs devices.
-
Ongoing: R&D continues in additive-manufactured titanium alloys, surface-textured implants, and smart sensors capable of monitoring healing progress.
About Us
DataM Intelligence is a leading provider of in-depth market research and consulting services, empowering clients to make strategic decisions through data-driven insights. Our multidisciplinary team delivers global coverage across healthcare, technology, chemicals, and other critical sectors.
Contact Us
DataM Intelligence
Email: info@datamintelligence.com
Phone: +1 877‑441‑4866
- Art
- Causes
- Crafts
- Dance
- Drinks
- Film
- Fitness
- Food
- משחקים
- Gardening
- Health
- בית
- Literature
- Music
- Networking
- לא משנה
- Party
- Religion
- Shopping
- Sports
- Theater
- Wellness