Idursulfase and Beyond: Therapeutic Advances in Hunter Syndrome
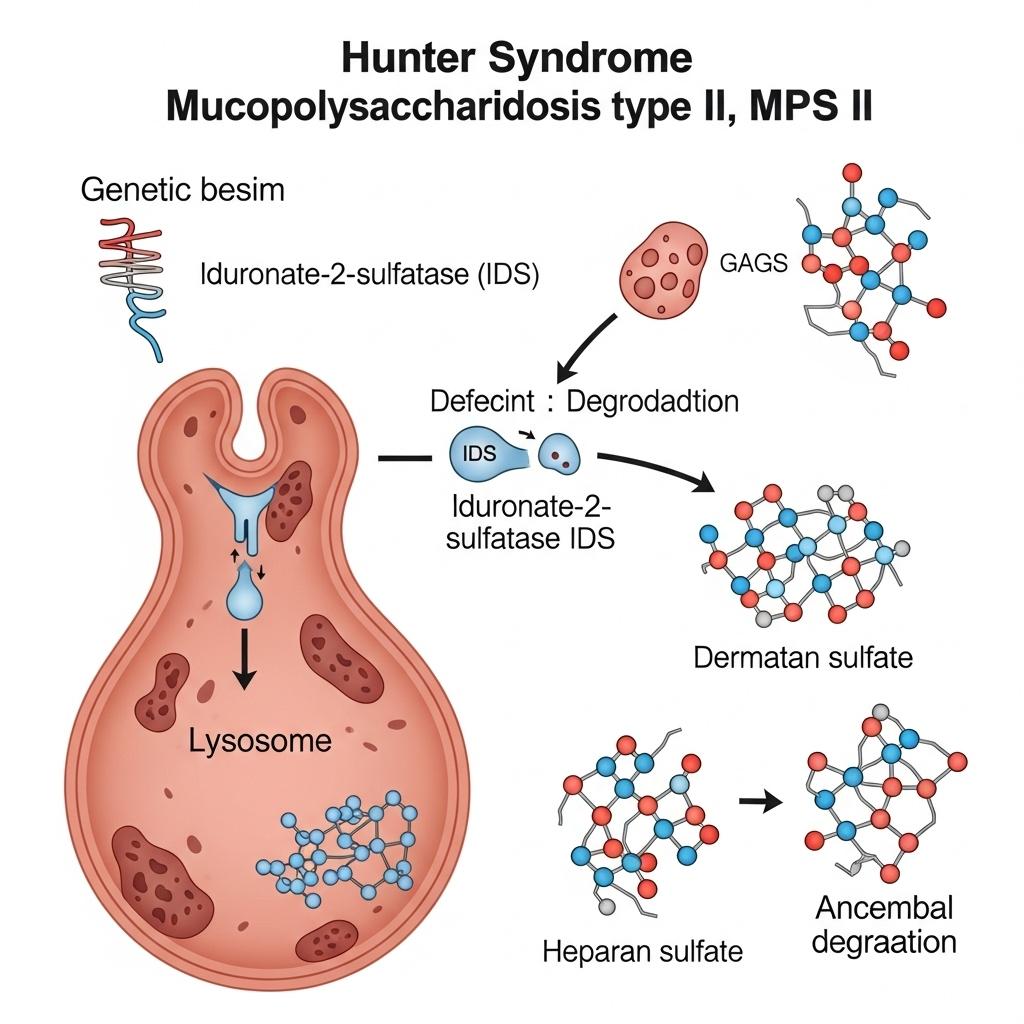
Hunter Syndrome, also known as Mucopolysaccharidosis Type II (MPS II), is a rare X-linked lysosomal storage disorder caused by mutations in the IDS gene, leading to a deficiency of the enzyme iduronate-2-sulfatase. This enzyme is critical for breaking down glycosaminoglycans (GAGs) like dermatan sulfate and heparan sulfate. Without it, GAGs accumulate in tissues, triggering progressive damage across multiple organs, including the brain in severe cases.
Primarily affecting males, MPS II is part of a larger group of mucopolysaccharidoses, but it is uniquely distinguished by its X-linked inheritance and high variability in clinical severity, from life-threatening early onset forms with cognitive decline to milder, somatic-only presentations.
Request a sample copy of the CI report at: https://www.datamintelligence.com/download-sample/hunter-syndrome-treatment-market
Clinical Spectrum: Two Phenotypes, One Genetic Cause
MPS II manifests as a continuum, but it is generally categorized into:
1. Severe (Neuronopathic) Form: Onset before age 2–4, with progressive neurocognitive decline, behavioral challenges, skeletal deformities, hepatosplenomegaly, and cardiopulmonary issues. Life expectancy without treatment is typically below 15 years.
2. Attenuated Form: Later onset with spared cognition, but similar somatic complications such as joint stiffness, obstructive airway disease, and heart valve abnormalities. Patients can live into adulthood with supportive care.
3. Characteristic signs include coarse facial features, macrocephaly, recurrent ear infections, enlarged tongue, hernias, and short stature. Diagnosing early and initiating treatment promptly is critical to slowing disease progression.
Diagnosis and Newborn Screening
Diagnosis involves:
1. Urinary GAG ***ysis: Elevated dermatan and heparan sulfate.
2. Enzyme Assay: Demonstrates reduced or absent iduronate-2-sulfatase activity in leukocytes or fibroblasts.
3. Molecular Genetic Testing: Confirms IDS gene mutations and supports family counseling.
4. Newborn screening is not yet universally adopted but is under pilot implementation in several regions due to improved therapeutic outcomes with early diagnosis.
Therapeutic Approaches
1. Enzyme Replacement Therapy (ERT)
Idursulfase (Elaprase®) is the only approved ERT for MPS II, administered via weekly IV infusions.
It significantly improves pulmonary function, mobility, and organomegaly, but it does not cross the blood-brain barrier (BBB), leaving CNS symptoms untreated in severe forms.
A newer formulation, pabinafusp alfa (Izcargo®), approved in Japan and undergoing global development, is ***gned to cross the BBB and improve neurological outcomes.
2. Supportive Care
Cardiac monitoring, ENT interventions, orthopedic surgeries, and respiratory support are often needed.
Educational and behavioral support are essential for patients with cognitive involvement.
Emerging Gene and CNS-Directed Therapies
A major goal in MPS II research is addressing CNS symptoms.
Promising investigational therapies include:
1. Gene Therapy: Trials such as those by Regenxbio and Sangamo explore AAV vector-based gene delivery, aiming for durable enzyme expression and improved systemic and neurological outcomes.
2. Intrathecal ERT: Trials are evaluating direct cerebrospinal fluid delivery to enhance CNS exposure.
3. Substrate Reduction Therapy (SRT) and chaperone therapies are in preclinical or early-phase development, aiming to reduce GAG production or enhance enzyme stability.
These approaches represent a paradigm shift from symptom control to potential disease modification.
Read the full CI Insights report: https://www.datamintelligence.com/strategic-insights/hunter-syndrome-mucopolysaccharidosis-type-ii-mps-i
Challenges and Global Perspectives
The limited CNS benefit of current ERT remains a core unmet need in severe MPS II.
Global access to therapies and diagnostics is uneven, particularly in low-resource settings.
High treatment costs and lifelong infusion burdens raise concerns around sustainability and adherence.
Multidisciplinary management and clinical trial participation offer patients improved outcomes and access to cutting-edge care.
About DataM Intelligence
DataM Intelligence 4Market Research LLP delivers real-time competitive intelligence across autoimmune, immunologic, and rare disease spaces. Our insights span clinical pipelines, regulatory benchmarks, and commercialization strategies for stakeholders in global life sciences.
🔗 Visit: www.datamintelligence.com
- Art
- Causes
- Crafts
- Dance
- Drinks
- Film
- Fitness
- Food
- الألعاب
- Gardening
- Health
- الرئيسية
- Literature
- Music
- Networking
- أخرى
- Party
- Religion
- Shopping
- Sports
- Theater
- Wellness


