Laronidase, HSCT, and the Future of MPS I Management
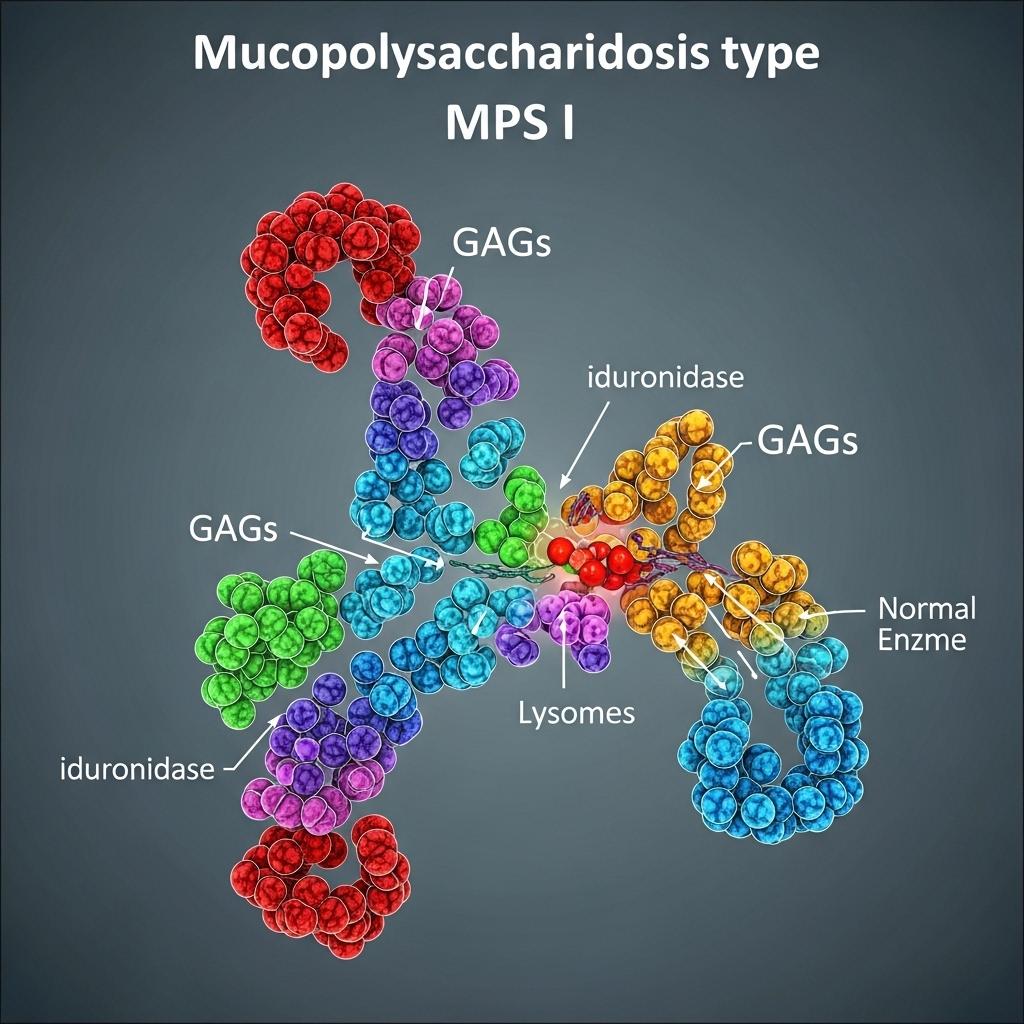
Mucopolysaccharidosis Type I (MPS I) is a rare, inherited lysosomal storage disorder caused by mutations in the IDUA gene, which encodes the enzyme alpha-L-iduronidase. This enzyme is responsible for breaking down glycosaminoglycans (GAGs), including dermatan sulfate and heparan sulfate. In MPS I, deficiency or absence of alpha-L-iduronidase leads to progressive accumulation of GAGs in various tissues and organs, resulting in multisystemic manifestations.
MPS I encompasses a clinical spectrum ranging from the severe Hurler syndrome to the more attenuated Hurler–Scheie and Scheie syndromes. Though historically underdiagnosed, advancements in genetic screening and newborn testing are improving early identification and management of this life-limiting condition.
Request a sample copy of the CI report at:
https://www.datamintelligence.com/download-sample/mucopolysaccharidosis-treatment-market
Clinical Spectrum: Hurler to Scheie
The phenotype of MPS I varies depending on residual enzyme activity:
* Hurler Syndrome (MPS I-H): Severe form with early-onset neurodegeneration, skeletal abnormalities, corneal clouding, and cardiopulmonary decline. Life expectancy without treatment is often under 10 years.
* Hurler–Scheie (MPS I-H/S): Intermediate severity with some neurological involvement, joint stiffness, and organ dysfunction.
* Scheie Syndrome (MPS I-S): Attenuated form with later onset, normal intelligence, and primarily somatic features like joint contractures, hernias, and cardiac valve disease.
Common features include coarse facial features, hepatosplenomegaly, respiratory issues, hearing loss, and restricted mobility. Without early intervention, progressive deterioration affects quality and duration of life.
Diagnostic Approaches and Newborn Screening
Early diagnosis is crucial for optimal outcomes. Testing typically includes:
* Enzyme assay: Measures alpha-L-iduronidase activity in leukocytes or fibroblasts.
* Urine GAG ***ysis: Elevated dermatan and heparan sulfate.
* Genetic testing: Confirms IDUA mutations and aids in prognosis and family counseling.
* Newborn screening programs have been implemented in several countries, facilitating early treatment before irreversible damage occurs.
Current Therapeutic Landscape
1. Enzyme Replacement Therapy (ERT)
Laronidase (Aldurazyme®) is the first and only approved ERT for MPS I. Administered intravenously, it improves pulmonary function, mobility, and reduces hepatosplenomegaly. However, ERT has limited penetration across the blood–brain barrier (BBB), making it insufficient for central nervous system (CNS) symptoms.
2. Hematopoietic Stem Cell Transplantation (HSCT)
For infants with Hurler syndrome, HSCT is the standard of care when performed early. It provides donor-derived cells capable of producing the missing enzyme, potentially halting neurocognitive decline. However, risks include graft-versus-host disease, graft failure, and transplant-related mortality.
Emerging and Investigational Therapies
A wave of innovation is underway to address ERT limitations and target CNS symptoms:
1. Intrathecal ERT: Trials are ongoing to evaluate direct delivery of laronidase into the cerebrospinal fluid to bypass the BBB.
2. Gene Therapy: Several platforms are in development, including:
AAV-based gene therapy (e.g., REGENXBIO’s RGX-111): Delivers functional IDUA gene into the CNS via int***ernal injection.
3. Lenti*** gene therapy: Involves autologous stem cell modification and re-infusion, potentially providing systemic and CNS benefits.
4. Substrate Reduction Therapy (SRT): Small molecules to reduce GAG synthesis or enhance lysosomal clearance are in preclinical exploration.
These approaches aim for durable enzyme expression, neurological benefit, and reduced treatment burden.
Read the full CI Insights report: https://www.datamintelligence.com/strategic-insights/mucopolysaccharidosis-type-i-mps-i-treatment-revolution-the-role-of-innovative-gene-therapy-in-addressing-unmet-needs
Real-World Challenges and Future Directions
1. Access to HSCT and ERT remains inconsistent across regions, especially in low-resource countries.
2. Lifelong infusion burdens and the absence of CNS protection in attenuated forms drive unmet needs.
3. Long-term follow-up data are essential to understand ERT durability and gene therapy safety.
4. Looking forward, a combination of early screening, genotype-based treatment planning, and next-generation therapies will define the future standard of care.
About DataM Intelligence
DataM Intelligence 4Market Research LLP delivers real-time competitive intelligence across autoimmune, immunologic, and rare disease landscapes. Our insights support clinical development, regulatory navigation, and market strategy across the global life sciences ecosystem.
🔗 Visit: www.datamintelligence.com
- Art
- Causes
- Crafts
- Dance
- Drinks
- Film
- Fitness
- Food
- Игры
- Gardening
- Health
- Главная
- Literature
- Music
- Networking
- Другое
- Party
- Religion
- Shopping
- Sports
- Theater
- Wellness


