إعلان مُمول
Japan Market to Grow 22% CAGR Through 2033, India Joins Race
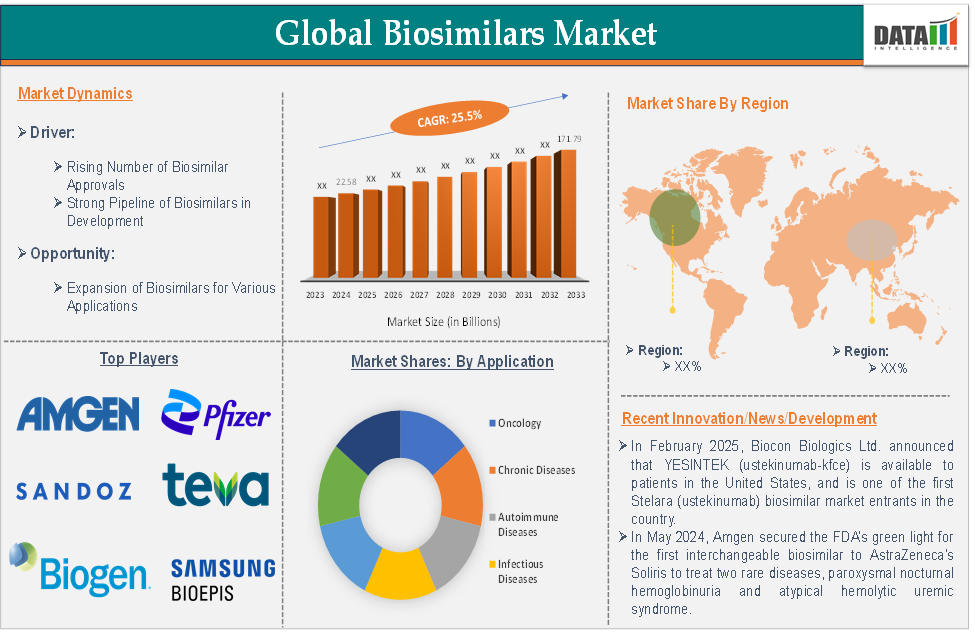
The biosimilars market—offering cost-effective, near-biologic therapies—is set for rapid expansion. India’s growing role complements major global momentum, driven by patent expirations, chronic disease prevalence, and supportive regulatory frameworks worldwide.
Biosimilars-market
Request a sample copy of research report:https://www.datamintelligence.com/download-sample/biosimilars-market
Powerful Market Momentum
-
Global Growth Forecast
The global biosimilars market was valued at USD 24 billion in 2024, with an expected CAGR of 19.6% to 2031, reaching USD 84 billion by then. -
Asia‑Pacific Leader
The Asia-Pacific segment accounted for USD 9.95 billion in 2024 and is projected to surpass USD 55 billion by 2032 (CAGR ***7%). India is the region’s fastest-growing market, expected to hit USD 2.725 billion by 2027.
India’s Strategic Biosimilar Rise
-
Domestic Market Outlook
Though exact current data from DataM Intelligence is proprietary, industry sources estimate India’s biosimilar sector will reach USD 35 billion by 2030, reflecting surging domestic R&D and biosimilar manufacturing. -
Industry Foundations
India boasts 670-plus USFDA-approved facilities, produces nearly half of global vaccine demand, and supports top biosimilar firms: Biocon Biologics, Intas, Dr. Reddy’s, Cipla, Aurobindo, Reliance Lifesciences. - Competitive Consolidation
M&A and strategic alliances (e.g., Abbott–mAbxience, Biocon–Sandoz) are consolidating expertise and accelerating launches.
Key Drivers & Market Dynamics
-
Patent Expirations Unlocking Markets
Loss of patents on blockbuster biologics—such as Herceptin, Rituxan, Enbrel, Humira and Stelara—fuels biosimilar entry. For example, Celltrion's CT-P43 (Stelara biosimilar) is set to enter the US in March 2025. -
Cost Savings & Access
Biosimilars cost 30–70% less than originators—e.g. Remicade’s vial at USD 500 vs. USD 1,600 for original—leading to potential savings up to USD 38 billion in US healthcare spend (2021–25). -
Chronic Diseases & Aging Demographics
Rising chronic disease burden (cancer, autoimmune, diabetes) and global aging populations are expanding biosimilar demand—even more pronounced in Japan with its geriatric demographics. -
Regulatory & Market Evolution
Streamlining by FDA, PMDA (Japan), and EMA—like BPCIA in the US (since 2010) and PMDA reforms—shortens market entry time. -
Competitive Consolidation
M&A and strategic alliances (e.g., Abbott–mAbxience, Biocon–Sandoz) are consolidating expertise and accelerating launches.
Trends in the US & Japan
United States
-
Retail & PBM Shifts
CVS’s launch of Cordavis and its biosimilar Hyrimoz (Humira biosimilar) reflects payor strategy shifts. US biosimilars projected at <USD 10B in 2022 to over USD 100B by 2029. -
Regulatory Expansion
As of October 2024, the FDA has approved 60 biosimilars. Under BPCIA, biosimilars are now interchangeable for prescriptions, reducing time and cost.
Japan
-
*** Growth
Japan’s market grew from USD 314.6 million in 2020 to USD 1.268 billion by 2027 (CAGR 22%). -
Domestic Surge
IMARC data forecasts growth from USD 475.8 million in 2024 to USD 3.42 billion by 2033 (CAGR 22.7%). -
Regulatory & Reimbursement Support
PMDA’s streamlined approvals, alignment with EMA/FDA pathways, and generous reimbursement drive rising biosimilar adoption in elderly care.
India-Specific Growth Opportunities
-
Chronic Disease Burden & Cost Pressure
India's rising diabetes, cancer, and autoimmune cases mirror global patterns—biosimilars can alleviate patient and government spending. -
Government & Regulatory Push
With supportive policies and evolving domestic approvals, India is poised to deepen its biosimilar space. -
Export Potential
Scaled production and quality, backed by USFDA production sites, strengthen India’s role as a global exporter of biosimilars and biologics. -
Domestic Innovation
Startups and biotech INR 25,000 cr government funding are developing next-gen biosimilars and contract research/manufacturing (CRDMO) services. -
Strategic Collaborations
Partnerships (e.g., Biocon–Sandoz, Abbott–mAbxience) and expanding portfolios (e.g. oncology, immunology biosimilars) will increase India's global competitiveness.
Market Challenges
-
Manufacturing Complexity & Immunogenicity
Biosimilars require expensive, high-precision processes, adding to development risk. -
Physician & Patient Resistance
Lack of familiarity and perceived safety concerns slow adoption—especially in Japan . -
Pricing Pressure
Fierce competition leads to shrinking margins, as manufacturers seek differentiation beyond cost savings . -
PBM Strategies in US
Rebates and formularies can favor originators over biosimilars, complicating market entry .
Expert Opinion
“Patent expirations create lucrative biosimilar opportunities in oncology and autoimmune spaces,” notes Global Research Firm.
“Asia‑Pacific is the fastest-growing region, with India leading the charge thanks to expanded biosimilar production and affordable pricing,” says MarketsandMarkets.
Request a Quotation: https://www.datamintelligence.com/buy-now-page?report=biosimilars-market
Future Outlook
The biosimilars market is at a pivotal growth juncture. For India, aligning domestic capacity with regulatory support and global partnerships promises millions in healthcare savings and enhanced treatment access.
Strategic Recommendations:
-
Policymakers & Regulators should enhance interchangeability rules and reimbursement models.
-
Manufacturers must invest in high-quality production, biosimilar differentiation, and therapeutic innovation.
-
Healthcare Providers should be educated to build trust and expand biosimilar use.
-
Global Investors have opportunity in India’s rising biosimilar and biopharma sectors—propelled by CRO/CMO capabilities and export scale




